I was inspired by a segment on the first show of Science Friday’s Cephalopod Week when Dr. Janet Voight (12:11 – 13:44) casually mentioned how the Curled Octopus envenomates its crustacean prey.
Directly to the eyeball.
Terrifying as that is (imagine the last thing you ever see are jaws puncturing your eyes, leaving you blind and paralyzed), this is a pretty effective way to get venom right where it can do the most damage. Stinging through the eyestalk puts these toxins into the brain and directly into the body cavity of your prey. More bang for the buck when it comes to incapacitating prey. There is evidence their venom provides a little pre-digestion of the tissues attaching the hard outside of their crabby dinners. Grisley et al (1996) described this eye-puncturing behavior as a key innovation in breaking down tissues holding together the crab carapace to “immobilize an otherwise active and aggressive adversary.” From the crabbing I have done with my family, I totally understand the need to get these animals to cool it quickly. Linked is a video of a curled octopus snagging dinner.


Octopus venom is transmitted through the saliva in posterior salivary glands. Initially, scientists thought toxins in octopus saliva traveled through the water and eventually entered through the crab’s gills (Fig 1, Fry et al 2009). But crabs exposed to saliva-containing water were not affected, even when “several legs had been torn away” (Grisley et al 1996). That sounds like a classic grad student job, pulling the legs off an angry crab. The crabs injected with saliva died within minutes, every single time, which is a clear signal that this mixture is venomous. This specialized method of injecting toxins is just one of the hunting strategies that cephalopods use to capture prey (Villaneuva et al 2017). Others will bore holes directly into the carapace or pull apart their prey. Injecting venom through the eye appears to be a technique used by smaller octopuses. Larger animals may not need to use venom to incapacitate their prey. The physical action of boring a hole or pulling their prey apart is probably enough.
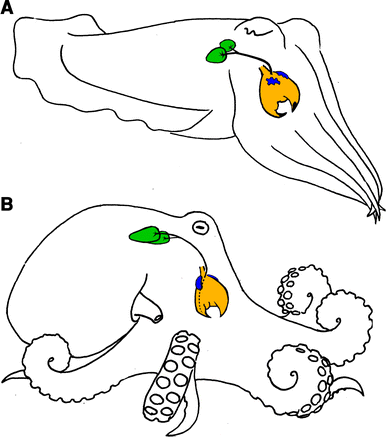
However, venom does appear to be an old method used by cephalopods. Fry et al (2009) studied the venom glands of three species of cephalopods representing three distinct families and found venom genes appear to have been recruited early in the cephalopod lineage. Even more surprisingly (and probably more impactful for venom researchers), this study found that mollusks (animal phylum) appear to display similar evolutionary recruitment of venoms as other distant lineages. In other words, venoms genes evolved in cephalopods from similar protein families as have been described in reptilian and insect venoms. Convergent evolution is when certain traits of similar function evolve in multiple linages without having been present in a common ancestor. In this case, venom was not present in the ancestor to all animals but rather arose independently multiple times within animals. Venom is an extraordinary example of convergence and has evolved nearly 90 separate times within various animal phyla. Fry et al (2009) found the genes that appear to have given rise to venoms in cephalopods, non-venomous housekeeping genes, are similar to those that give rise to venoms in reptiles and insects. This indicates an evolutionary constraint, or limitation on the types of genes that have the potential to adapt into a venomous component. That limitation may be similar across the vast diversity of the animal kingdom, which is valuable to understanding animal evolution more generally.
Octopod venom also provides a chance to look at how the environment influences venom composition, such as cold-adapted venoms from Antarctic octopuses. Undheim et al (2010) found the venom components of Antarctic octopuses were remarkably similar to those of the more temperate and tropical species, and only a few components appear to be specifically adapted to the cold. Cold adaptive venoms could be invaluable in the search of new drugs since these compounds persist in harsher conditions. Additionally, the composition of these venoms could provide more clues into the diets of these animals, which would provide more ecological information about poorly understood species. There were several morphological characteristics that suggested adaption to physical venom delivery; larger posterior salivary glands and smaller beaks, potentially enabling faster delivery of more toxins to prey. Like their more temperate cousins that that inject venoms into the eyes of crabs, these animals have adapted to use their toxic weaponry in a specialized way.
The Curled Octopuses is not the only example of a carefully placed sting to the brain. Jewel wasps (mentioned briefly in the Venom vs Poison article) are beautiful insects that take their venom to the extreme side of manipulation in their parasitism of cockroaches. Below is a video of the dreadful dance. The female jewel wasp’s first sting paralyzes the roach’s legs, then she stings right into a specific region of the cockroach’s brain, giving the wasp full control of the animal. The roach proceeds to clean itself obsessively, allows the wasp to break its antenna and drink some nourishing blood. She then leads the roach by the broken antenna into a hole the wasp has dug, lays an egg on the roach, and buries the roach alive. Then the roach is consumed by the hatched wasp, alive. Yes, this exists.
In my own research, one of the major questions I am hoping to work in is what components of jellyfish venom is used in digestion or pre-digestion versus, since these gelatinous animals also consume much heartier, chitinous prey. This eyestalk strategy used by octopuses made me think about a recent article exploring the proteome of the gem anemone, Bunodactis verrucose. Domínguez-Pérez et al (2018) proposed that because mussels appear to be the main diet of this anemone, some of the venom proteins they found may be used synergistically to open the shells of their mussel prey. Proteases break down the tissue sealing the mussel’s shell, allowing the neurotoxic components of the venom to invade and act on the adductor muscle holding the shell tightly closely. Together these components give anemones the ability to break open their prey to consume the tissue inside. One of those neurotoxic components found in this anemone is a SE-cephalotoxin. CEPHALOtoxin. You guessed it, a similar kind of toxin purified from octopuses used to subdue crabs and other bivalves, emphasizing how similar environments can result in convergence in venom composition and utility. Venom is the ultimate case of if it works, don’t fix it.

References
- Beautiful wasp zombifies cockroach. (2015). YouTube. Retrieved from https://www.youtube.com/watch?v=-ySwuQhruBo
- Cephalopod Week Jets Off For 2018. Science Friday. Retrieved from www.sciencefriday.com/segments/scifris-tentacled-spectacle-cephalopod-week-returns/
- Octopus Eats Crab. (2014). YouTube. Retrieved from https://youtu.be/kAal__PwwOo
- Domínguez-Pérez, D., Campos, A., Alexei Rodríguez, A., Turkina, M. V., Ribeiro, T., Osorio, H., … & Antunes, A. (2018). Proteomic Analyses of the Unexplored Sea Anemone Bunodactis verrucosa. Marine drugs, 16(2), 42.
- Fry, B. G., Roelants, K., & Norman, J. A. (2009). Tentacles of venom: toxic protein convergence in the Kingdom Animalia. Journal of molecular evolution, 68(4), 311-321.
- Grisley, M. S., Boyle, P. R., & Key, L. N. (1996). Eye puncture as a route of entry for saliva during predation on crabs by the octopus Eledone cirrhosa (Lamarck). Journal of Experimental Marine Biology and Ecology, 202(2), 225-237.
- Undheim, E. A. B., Georgieva, D. N., Thoen, H. H., Norman, J. A., Mork, J., Betzel, C., & Fry, B. G. (2010). Venom on ice: First insights into Antarctic octopus venoms. Toxicon, 56(6), 897-913.
- Villanueva, R., Perricone, V., & Fiorito, G. (2017). Cephalopods as predators: a short journey among behavioral flexibilities, adaptions, and feeding habits. Frontiers in physiology, 8, 598.

Nice
Sent from my lazyboy
>
LikeLike